Healthcare Scanner
3D shape trade marks for medical devices
Juni 2022
Innovation in medical devices continues strongly and the introduction of IoT and 3D printing (amongst others) has led to an acceleration in new medical devices. Whilst patents and designs are the obvious routes to protect medical devices, consideration should also be given to 3D shape trade marks.
How to protect
Trade marks: Although trade marks are often seen as protecting the brand name and reputation, it is possible to register 3D shapes if they also function as a brand. This is because the legal definition of a trade mark is “any sign which is capable:
(a) of being represented in the register in a manner which enables the registrar and other competent authorities and the public to determine the clear and precise subject matter of the protection afforded to the proprietor, and
(b) of distinguishing goods or services of one undertaking from those of other undertakings.”
A registered trade mark grants the owner a monopoly right in their mark for an indefinite period of time (subject to renewal and being able to keep the registration in force through use of the mark). Registration should be considered as it can be a powerful protection and enforcement tool.
There are a number of key factors that limit the registrability of 3D shape trade marks. The main factors that limit the registration are if the shape:
(a) arises from the inherent nature of the product/goods
(b) provides a technical function
(c) adds substantial value to the product.
Problems with shape trade marks
A number of problems arise in both achieving registration and enforcement of the rights. The EU’s General Court and the Court of Justice of the European Union have held in a number of cases that only shape marks which depart significantly from the norm and customs in the sector are not devoid of distinctive character.
Part of the issue is that consumers are not accustomed to identifying 3D shapes as brand identifiers in the same way that they are accustomed to identifying brand names, logos or slogans. An inhaler or diabetic pump, for example, with the brand name included provides the consumer instantly with information as to the trade origin of the goods. The same products, without brand indications, are unusual in actual use.
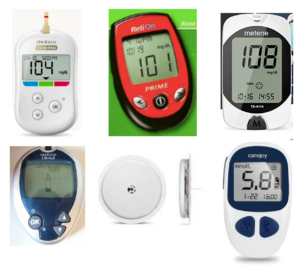
Some products may simply all look like others in the sector. A (not very scientific) internet search for “pacemakers”, revealed (amongst others) the following results:
The devices all follow a similar shape, although there are some small differences. As such, a company seeking to register such a device as a three dimensional trade mark would need to ensure that the device (or a component thereof) departs significantly from the norms of the sector.
Products with a degree of “design freedom” are also more likely to be seen as different or distinctive in the marketplace. A similar internet search for “blood sugar testing kits” shows that there is a lot more variety of shapes, colours and variables available, and arguably, such devices would be ‘easier’ to register as three dimensional trade marks.
Evidence of acquired distinctiveness
Evidence of “acquired distinctiveness” can be very useful in overcoming distinctiveness objections in the examination of a 3D shape mark, showing that consumers have been educated to perceive the product shape as originating from a particular undertaking. Such evidence can include market share, sales figures, as well as advertising figures, enforcement of rights against third parties, and surveys showing consumers associate the shape with the producer without reliance on any other brand identifiers (such as a brand name).
However, it should be noted that no level of evidence will be sufficient to overcome objections raised in relation to the three exclusions (a) – (c) noted above.
Other “non-traditional” mark types
Not all filings need to be three dimensional either. Some companies seek to register a particular element of a product (think Levi’s Red Tab on jeans), or a particular colour combination or colour of a specific part of a product (such as Louboutin’s red shoe soles). Also, only certain parts of a device may be distinctive, and images of these can be applied for separately from the device as a whole.
Examples on the UKIPO database
The following examples are taken from searches on the UKIPO registry database in Class 10.
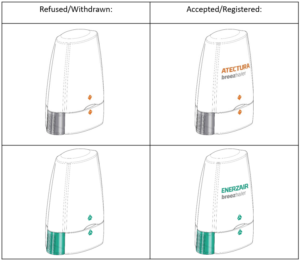
Novartis AG – inhalers
Whilst the images on the left include a design image, there is no brand name, and the applications appear to have received objections from the Registry and were subsequently withdrawn by Novartis. The same device, but filed with the brand name included, were accepted for registration.
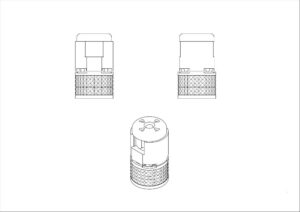
Vetter Pharma-Fetigung GmbH & Co. KG
Vetter has a pending application for a component part of an injection device in Class 10. The same image was applied for the EUIPO, and is also still pending. Without any background to the filings, it is safe to assume that an objection was raised by both the UK and EU registries on the basis that (1) the mark lacks any distinctive character and (2) that the characteristics of the mark are necessary to obtain a technical result.
If the technical objection has been raised, it would not be possible to overcome the distinctiveness objection, and instead, Vetter will need to seek to have the objection waived.
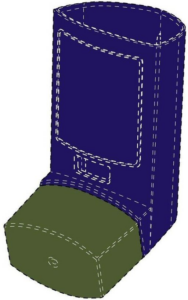
Lupin Atlantis Holdings SA
This example is a UK registration in Classes 5 and 10 in respect of inhalers. The mark is actually less of a 3D shape, but covers more the colour combination of blue/violet and green. This is indicated by the dotted lines outlining the product shape. However, it would be interesting to see the validity of such a registration, as the CJEU has previously stated in case law that it is irrelevant whether the mark is regarded as a position, figurative or colour mark; it relates to the application of three dimensional elements to the surface of a product and can be assessed as such alongside any evidence of actual genuine use.
Colour is claimed as part of the registration: the color blue violet applied to the actuator of an inhaler, and the color green applied to the cap of an inhaler.
STADA Arzneimittel AG
This example is actually a cloned UK registration from an EUIPO registration upon Brexit and refers more to a “position” mark than a straightforward 3D shape. The mark does include the brand name “STADA”, but the dotted lines indicate that the only element that STADA seek to register is the pink corner element on the left together with the STADA logo on the right.
CELON PHARMA Spółka Akcyjna (S.A.)
This example is another cloned UK registration, but for a 3D shape of a multi-dose inhalation product in Classes 5 and 10. Although registered at the EUIPO originally, it appears the mark was accepted for registration without issue, taking only five months from filing to proceed to registration.
Invalidation risk
Even if brand owners get over the first hurdle and obtain registration for a 3D shape mark, there are continued difficulties in maintaining the protection, especially when seeking to enforce. A common counter-attack in infringement proceedings is a claim that the registration is invalid as the product is functional or necessary to obtain a technical result.
Anti-counterfeiting considerations
Once a medical device has launched on the market, third parties will inevitably be tempted to copy it. The IP associated with the device should be registered with customs authorities in the relevant territories, which will enable customs officials to detain and potentially destroy infringing items.
Enforcement
Brand practices need to be established to assess how infringements will be tackled. There are numerous providers of monitoring systems which will assist in identifying potential infringements. Procedures for dealing with such actions will be important, both for keeping the market place clear and for evidence of such enforcement in the event of incoming threats of infringement.
Next steps
As can be seen, there are a number of considerations to take into account, but trade mark protection for medical devices can be a useful protection tool.
Some of the examples above show the different types of marks for which protection can be sought, and some of the difficulties that can be encountered. When looking to file trade marks, it can be useful to consider different variations that can be filed – for example, variations with and without the brand name, position marks or colour combinations. This not only increases the chances of achieving registration, but potentially also in terms of validity of the resulting registrations if challenged.
This article was prepared by HGF’s Trade Mark Director Claire Jones.