Articles
The Patentability of Deuterated Pharmaceuticals: Insights from the EPO
Januar 2026
Deuteration, also known as the “deuterium switch”, refers to the substitution of protium (¹H) for its heavier isotope, deuterium (²H, or D), within a molecular structure. Due to the strength of the C-D bond this modification can lead to improvements in a drug’s pharmacological properties, pharmacokinetic profile, and toxicity.[1]
Deuteration, therefore, represents a powerful tool not only for enhancing the properties of existing drugs but also for supporting the discovery and development of new pharmaceuticals. The deuterated drugs market size was valued at $368.6 million in 2024 and is estimated to reach $795.7 million by 2031.[2]
However, as the field continues to expand, a pertinent question arises: To what extent are deuterated pharmaceuticals patentable?
Since the potential benefits of deuteration are well understood, de novo deuteration is becoming an increasingly integral aspect of pharmaceutical development. However, the deuteration of known active pharmaceutical compounds offers clear cost and time advantages over de novo drug design. This article addresses patentability issues of deuterated pharmaceuticals in Europe, where the non-deuterated parent compound already forms part of the state of the art.
Although there is a lack of European case law regarding the patentability of deuterated pharmaceuticals, an analysis of the prosecution of several applications before the Examining Division (ED) of the European Patent Office (EPO), along with two decisions by the more authoritative Opposition Division (OD), offers insight into the EPO’s position and provides guidance for applicants seeking patent protection for deuterated compounds.[3]
Novelty
To be patentable before the EPO, a claimed invention must be both novel and inventive.
A claimed deuterated compound will generally be deemed novel so long as the specifically claimed deuterated form of that compound was not publicly disclosed before the filing date of the patent application.
It may be that a previously published patent application includes a general statement that aims to cover any deuterated analogue of a compound disclosed therein. Where the compound contains multiple positions that could be deuterated, such a general statement should not destroy the novelty of a subsequently claimed specifically deuterated analogue. However, if the compound possesses only a single possible site for deuteration, a general statement covering deuterated analogues may destroy the novelty of a specific deuterated compound.
Since deuterium occurs naturally at low abundance, trace amounts of various deuterated isotopologues will be present in a sample of the previously disclosed “non‑deuterated” parent compound. Even if it were statistically likely that the sample contained a particular claimed deuterated compound, under EPO practice this would typically not constitute a novelty‑destroying disclosure, as such a sample would generally be treated as lacking any direct and unambiguous disclosure of that specific deuterated compound. Nevertheless, it remains good practice to include language in a patent specification to explain that a position designated as “D” in a chemical structure would be understood by a skilled person to be occupied by deuterium at an isotopic abundance above its natural level, followed by fallbacks that allow higher isotopic purities to be defined, e.g., greater than 50%, 90%, 95%, and so on.
Inventive step
When assessing inventiveness, the EPO will consider what “technical effect” is achieved by a claimed deuterated compound over the closest prior art, typically the non-deuterated parent compound. In the majority of cases, such a technical effect is established by providing data demonstrating that the claimed compound offers some advantage over the non-deuterated parent compound. In the absence of such data, the EPO is likely to conclude that no technical effect is achieved and, consequently, that the claimed compound is an obvious alternative to the parent. For example, in EP 3810134 A1, CeleCor Therapeutics failed to provide data showing that the claimed deuterated RUC-4 (zalunfiban) derivatives offered any improvement over zalunfiban. As no technical advantage was demonstrated, the EPO could not recognise an inventive step. The application was subsequently withdrawn.
Even if a technical effect is established, e.g., via the provision of data, the EPO will still ask whether such a technical effect would have been obvious in light of the prior art at hand and the skilled person’s common general knowledge. The following cases illustrate how this assessment can vary depending on both the supporting data and the nature of the prior art landscape.
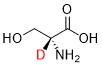
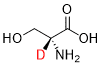
EP 3713557 B1 (Sun Pharmaceutical Industries) was granted with claims directed to a pharmaceutical composition comprising D-serine deuterated at the α-carbon:
The application included data showing that this analogue retained similar in vitro NMDA receptor binding affinity, while exhibiting advantageous pharmacokinetic properties and reduced nephrotoxicity, compared to non-deuterated D-serine.
The ED found the claimed compound to be inventive, noting that although it was well known at the time of filing that selective deuteration of bioactive compounds can, in some cases, improve safety, efficacy, or tolerability, the magnitude and occurrence of such benefits are inherently unpredictable, requiring individual assessment. In this case, the observed beneficial properties were deemed unpredictable and thus gave rise to an inventive step.
This view was also shared by the OD in the opposition to Vertex Pharmaceuticals’ patent EP 3352757 B1, relating to the second medical use of a deuterated ivacaftor analogue:
The OD contended that while deuteration is a well-known strategy to reduce drug clearance and increase half-life, the effects of deuteration on a drug’s metabolic profile are unpredictable and must be evaluated on a case-by-case basis. The OD therefore concluded that the skilled person would not have reasonably expected the claimed deuteration to improve ivacaftor’s metabolic properties and pharmacokinetics, supporting the inventiveness of the claims. The opposition was ultimately dismissed.
The EPO’s reasoning in these two cases appears to reflect their general approach to assessing the inventiveness of deuterated compounds. However, as the following decisions illustrate, the analysis can be more nuanced depending on the specific facts and evidence at hand.
In EP 2326643 B1, Auspex Pharmaceuticals obtained protection for deutetrabenazine, the first FDA-approved deuterated drug. Structures of tetrabenazine and deutetrabenazine are provided below:
The ED initially rejected a broader claim set covering all deuterated forms of tetrabenazine. They argued that it was speculative to assume that each claimed compound would exhibit reduced metabolic degradation based on data provided solely for deutetrabenazine. Since the claims covered compounds that had not been tested, the EPO did not recognise an inventive step across the entire claimed scope, particularly as the general strategy of reducing metabolic degradation through deuteration was already known in the art.
In response, Auspex narrowed the claims to deutetrabenazine. They argued that deuteration near metabolic sites does not guarantee improved overall stability due to metabolic switching. This is where reduced metabolism at one site is offset by increased metabolism elsewhere. Thus, improved overall metabolic stability due to deuteration could not be predicted for compounds like tetrabenazine with multiple known metabolic pathways. However, the data in the application showed that specifically deuterating the methoxy groups of tetrabenazine improved overall metabolic stability. The EPO found this evidence and argument to be persuasive and granted the patent.
In a subsequent application, EP 2576552 A2, Auspex sought protection for dihydrotetrabenazine (HTBZ), an active metabolite of tetrabenazine, with the same methoxy deuteration as in deutetrabenazine.
However, the ED cited an FDA publication on tetrabenazine, which identified the methoxy groups as the principal metabolic sites. The ED argued that, starting from HTBZ, a skilled person would have had a reasonable expectation that methoxy deuteration would improve metabolic stability. Auspex’s arguments concerning metabolic switching were not found persuasive, and the claims were deemed to lack an inventive step. The application was subsequently withdrawn following a summons to oral proceedings and a negative preliminary opinion from the ED.
A key distinction between these two cases is that, in the latter, prior art had identified that metabolic deactivation occurred primarily via the methoxy groups of tetrabenazine. Such prior knowledge of metabolic pathways may therefore reduce the likelihood that a deuterated analogue is deemed inventive.
Janssen Pharmaceuticals were granted protection for d1-JNJ38877605 in EP 3227296 B1:
The compound is selectively deuterated at a key aldehyde oxidase metabolic site of the parent molecule.
Data in the application showed that this deuteration led to the formation of fewer undesirable metabolites associated with renal toxicity. Crucially, the data also showed that the deuteration triggered a metabolic switch, resulting in increased formation of an active metabolite, thereby allowing a lower therapeutically effective dose to achieve the desired c-Met inhibitory effect.
The ED concluded that this type of metabolic switch was not predictable from the prior art and therefore found d1-JNJ38877605 to be inventive.
This case highlights the unpredictability of metabolic switching triggered by deuteration. This unpredictability can support an inventive step, especially when the switch leads to a favourable effect, such as an increase in conversion to an active metabolite.
A Hinova Pharmaceuticals application[1] included data demonstrating that deuteration at certain positions of defactinib adversely affected its metabolic stability. This evidence supported Hinova’s position that, due to the complexity of metabolic processes, drug pharmacokinetics are influenced by multiple interacting factors and remain inherently unpredictable. As a result, claims directed to defactinib derivatives deuterated at other positions, which were shown to improve metabolic stability, were considered inventive, since the observed improvement in metabolic stability was deemed unexpected.
This case illustrates that “negative data” can accentuate the unexpected nature of an improvement in a given property resulting from deuteration at a specific position.
Immunomodulatory imide drugs (IMiDs), such as thalidomide, lenalidomide, pomalidomide, and avadomide, contain a chiral centre at the α-position relative to the carbonyl group of the glutarimide moiety. The hydrogen at this α-carbon chiral centre can be involved in enolization, which leads to interconversion between the sedative (R)-enantiomer and the teratogenic (S)-enantiomer.
Deuteria Pharmaceuticals filed an application[2] claiming pomalidomide deuterated at its chiral centre. The ED cited prior art demonstrating that deuteration at lenalidomide’s chiral centre slows epimerization and concluded that it would have been obvious to apply the same strategy to pomalidomide.
In 2018, DeuteRx’s European patent EP 2943201 B1 was opposed. The granted patent claimed avadomide deuterated at its chiral centre:
The compound is selectively deuterated at a key aldehyde oxidase metabolic site of the parent molecule.
Data in the application showed that this deuteration led to the formation of fewer undesirable metabolites associated with renal toxicity. Crucially, the data also showed that the deuteration triggered a metabolic switch, resulting in increased formation of an active metabolite, thereby allowing a lower therapeutically effective dose to achieve the desired c-Met inhibitory effect.
The ED concluded that this type of metabolic switch was not predictable from the prior art and therefore found d1-JNJ38877605 to be inventive.
This case highlights the unpredictability of metabolic switching triggered by deuteration. This unpredictability can support an inventive step, especially when the switch leads to a favourable effect, such as an increase in conversion to an active metabolite.
A Hinova Pharmaceuticals application[1] included data demonstrating that deuteration at certain positions of defactinib adversely affected its metabolic stability. This evidence supported Hinova’s position that, due to the complexity of metabolic processes, drug pharmacokinetics are influenced by multiple interacting factors and remain inherently unpredictable. As a result, claims directed to defactinib derivatives deuterated at other positions, which were shown to improve metabolic stability, were considered inventive, since the observed improvement in metabolic stability was deemed unexpected.
This case illustrates that “negative data” can accentuate the unexpected nature of an improvement in a given property resulting from deuteration at a specific position.
Immunomodulatory imide drugs (IMiDs), such as thalidomide, lenalidomide, pomalidomide, and avadomide, contain a chiral centre at the α-position relative to the carbonyl group of the glutarimide moiety. The hydrogen at this α-carbon chiral centre can be involved in enolization, which leads to interconversion between the sedative (R)-enantiomer and the teratogenic (S)-enantiomer.
Deuteria Pharmaceuticals filed an application[2] claiming pomalidomide deuterated at its chiral centre. The ED cited prior art demonstrating that deuteration at lenalidomide’s chiral centre slows epimerization and concluded that it would have been obvious to apply the same strategy to pomalidomide.
In 2018, DeuteRx’s European patent EP 2943201 B1 was opposed. The granted patent claimed avadomide deuterated at its chiral centre:
During opposition proceedings, the claims were limited to the (-)-enantiomer. The patent specification included data showing that the claimed compound was resistant to racemization at its chiral centre. Additionally, in vivo data demonstrated that this compound was more effective at inhibiting tumour growth than both a racemic mixture of avadomide and the corresponding deuterated (+)-enantiomer.
The opponent cited prior art on deuterated lenalidomide and thalidomide, arguing that deuteration at the chiral centre was known to slow racemization and favour the active enantiomer.
However, the OD considered the claim to be inventive, stating that in vivo efficacy cannot be reliably predicted and an improved absorption pattern does not necessarily translate into improved anti-tumour activity. They asserted that in vivo effects result from a complex interplay of multiple factors, and may vary significantly even with small chemical modifications. Accordingly, the OD concluded that the in vivo effect demonstrated for the claimed deuterated (-)-enantiomer constituted an inventive contribution over the prior art.
The decision illustrates that in vivo data demonstrating enhanced efficacy of a claimed deuterated compound may help establish inventiveness, even if it is known that deuteration might improve stability against stereochemical inversion or metabolism.
Although the decisions discussed above are from the European Patent Office, they are largely in line with the approach taken by the USPTO[1], as well as the decision by the Federal Circuit in Sun Pharm. v. Incyte[2]. In this decision, it was found that deuruxolitinib was obvious in view of a study on ruxolitinib, which showed that metabolism primarily occurred at the four methylene carbons of its cyclopentyl ring, the same positions deuterated in deuruxolitinib.
Summary
Deuterated derivatives of known pharmaceutical compounds are patentable in Europe. However, patentability must be assessed on a case-by-case basis, taking into account the available experimental data, the relevant prior art, and any preexisting knowledge regarding the lability of the parent compound. In light of the discussion above, key take-home points are as follows:
- A claimed deuterated compound will generally be considered novel over a disclosure of the corresponding non‑deuterated parent compound, even in cases where that disclosure contains a general statement that the parent compound may be deuterated.
- When assessing inventive step, the EPO is usually takes the view that, although deuteration is a known strategy for modifying the pharmacological properties of a drug, its effects are inherently unpredictable.
- Data demonstrating a technical benefit of a deuterated compound over its non-deuterated parent is, however, often crucial to demonstrate an inventive step.
- Knowledge of a metabolic soft spot may render it obvious to deuterate that position in order to improve metabolic stability.
- However, when multiple soft spots exist, it may be that an overall increase in metabolic stability is not obvious due to the potential for metabolic switching.
- Data showing that other deuterated variants fail to improve, or even adversely affect, a property relative to the parent compound may support inventive step.
- In vivo data demonstrating enhanced efficacy attributable to deuteration may further support inventive step, even where it might have been expected that deuteration at a labile position would improve stability.
For any questions relating to the above, please contact the authors, Harry Gregson, at hgregson@hgf.com, or Dan Woollaston, at dwoollaston@hgf.com.
[1] G. S. Timmins, Expert Opin. Ther. Patents, 2014, 24, 1067-1075
[2] U.S. Court of Appeals for the Federal Circuit, Sun Pharm. Indus., Inc. v. Incyte Corp., No. 2019-2011, Document No. 117 (Fed. Cir. Aug. 22, 2023)
[1] European patent application no. 19798903.1
[2] European patent application no. 11841603.1
[1] European patent application no. 19798903.1
[2] European patent application no. 11841603.1
[1] R. M. C. Di Martino, B. D. Maxwell and T. Pirali, Nat. Rev. Drug Discov., 2023, 22, 562–584
[2] https://www.precisionbusinessinsights.com/market-reports/deuterated-drugs-market
[3] Opinions issued by the Examining Division and Opposition Division may vary and do not set legal precedent. They are referenced here to illustrate the European Patent Office’s general approach to the assessment of inventive step for deuterated drugs. However, these opinions may not carry legal weight if relied upon as support in either prosecution or post-grant proceedings.
This article was prepared by Senior Patent Attorney Harry Gregson and Partner and Patent Attorney Dan Woollaston