News & Events
Latest updates

Regulatory Exclusivity for Medicinal Products in Europe
Video overview: New Medical Products 8 years of data exclusivity, followed by 2 years of market exclusivity. +1 year market exclusivity possible for a new indication showing significant clinical benefit. No exclusivity granted for new formulations or routes of …
Read article
Added Matter – Part 2
Video overview: Pre and Post-Grant Amendments are governed by Articles 123(2) & 123(3) EPC respectively Before grant, amendments can broaden the claim scope as long as the resulting claim is …
Read article
Added Matter – Part 1
Video overview: What is Added Matter? Added matter occurs when an amendment introduces content that goes beyond what was originally disclosed in the application as filed (Article 123(2) EPC). EPO …
Read article
Patenting Polymorphs at the EPO
Video overview: Novelty and Inventive Step Prior art must necessarily lead to the claimed polymorph to be novelty destroying Watch out for inherent disclosures in prior art—methods may implicitly produce …
Read article
Antibodies
Video overview: EPO Requirements for Antibody Applications The EPO Guidelines (G-II, 6) provide a standardised framework for antibody-related patent issues. Antibodies can be defined by various aspects, including but not …
Read article
Description Amendment Requirements at EPO
Video overview: At the final stage of prosecution, the EPO increasingly insists each patent description must align with the allowed claims to improve clarity and eliminate inconsistencies. However, this strict …
Read article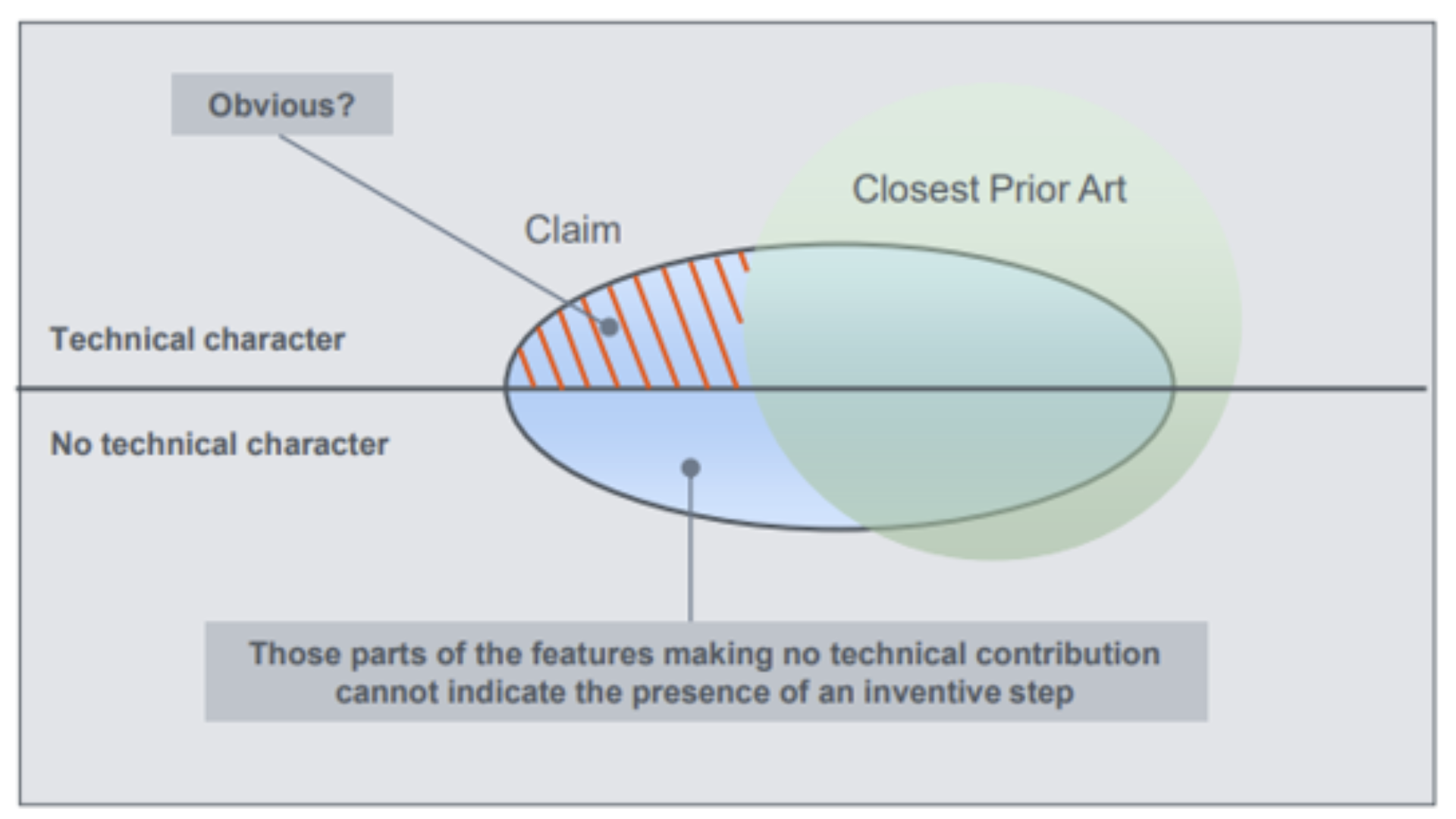
Computer-implemented Inventions
Video overview: To obtain a patent for a CII in Europe, two main hurdles must be cleared: Eligibility A claim must have technical character—even one technical feature is enough. Examples …
Read article
Bioinformatics at the EPO
Video overview: What is Bioinformatics? Bioinformatics involves the use of computational tools, software, algorithms, and AI to analyse biological data, such as genetic sequences, protein structures, or medical information. Are …
Read article
Claiming Priority
Video overview: To validly claim priority in a European patent application, four conditions must be met: Same Applicant or Successor in Title: The EP applicant must be the same as, …
Read article

























