Knowledge Hub
Comparing the value of European vs Unitary Patent Validation
November 2022
The EPO’s European patent system is on the brink of the biggest change in a generation – from 2023 it is expected that a new unitary patent (UP) will become available via the EPO.
The new Unitary Patent
Requesting a UP will create a single patent with unitary effect across all of the EU Member States (EU MS) that have ratified the UPC Agreement. Based on current ratifications, at launch the initial UP’s coverage will include at least 17 EU MS, including Germany, France, Italy and the Netherlands.
For future UPs, if all participating EU MS ratify the UPCA, the UP will cover 24 EU MS. But initially, the UP will cover the territories of countries that have ratified the UPC Agreement at the date of the registration of the unitary effect of the individual patent. Coverage of a UP will remain fixed throughout its lifetime and will not be extended to EU MS that join the UPC at a later date. The UP is subject entirely to the exclusive jurisdiction of the new UPC.
Costs vs coverage
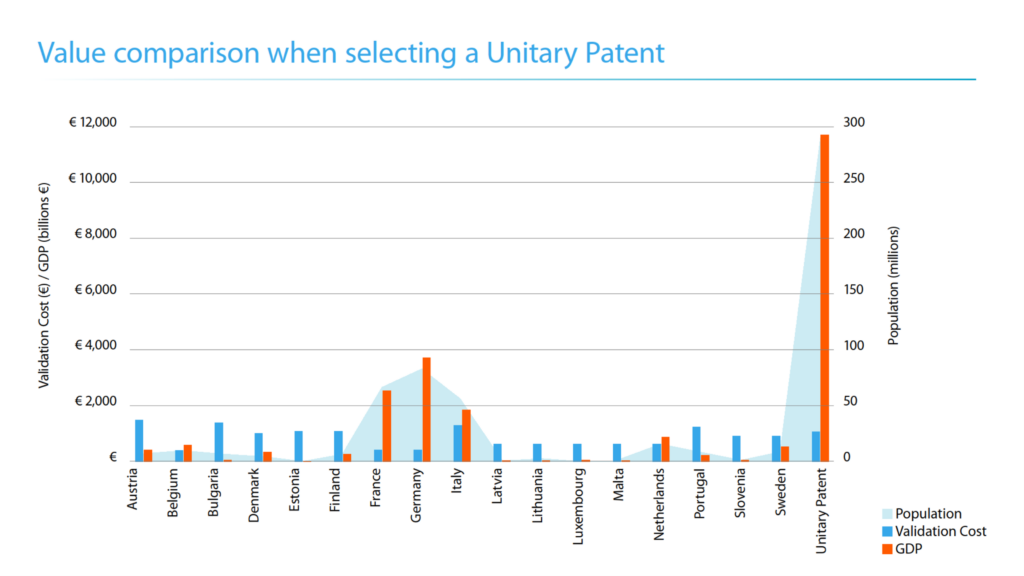
Renewal fees for a UP will be payable to the EPO in Euros. The EPO has set the renewal fees to be equivalent to the combined renewal fees that would be payable in the four countries where EPs were most often validated in 2015. Although the UP will be significantly more cost-effective than a widely-validated classical EP, because the UP is a unitary right, it is not possible to reduce the annual renewal fees by tapering the R-MS territories during the lifetime of the patent.
It is important to remember that, in order to obtain coverage in other EPC countries outside the UP at the time, patentees will still need to validate nationally in those EPC states.
So, will the UP be able to provide better value for patentees? Well, that depends!
In terms of validation costs relative to the population and GDP of the separate initial 17 UP EU MS, the UP presents excellent value as shown below. But in order to obtain coverage in other EPC countries outside the UP EU MS at the time, patentees will still need to validate nationally in those EPC states, which will be an additional cost on top of the UP itself.
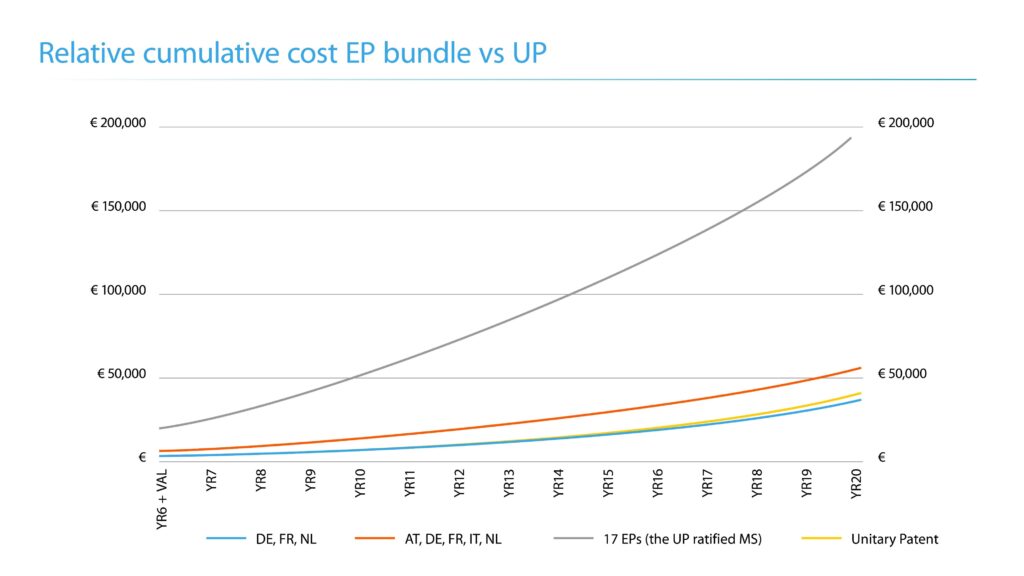
In terms of lifetime costs, the chart below shows the relative cumulative costs of an EP patent granted at year 6 (validation costs + renewal costs at year 6) and then the subsequent renewal costs from year 7 onwards, including official and professional fees. Assuming the patent was granted in year 6, validation and renewal costs become payable on grant. Depending on where the EP patent is validated, the cumulative costs will vary. A UP represents a substantial cost saving over the lifetime of the patent compared to separately validated EPs in 4 or more UP countries, however, it is important to remember that as a unitary right, a UP patent must be renewed as a whole, whereas EP patents validated may be renewed, or not, separately.
These figures have been shown as a guide for a specification filed in English having 15 claims.
If you would like to look at the relative costs of an EP that is close to grant, HGF can provide a detailed cost estimate for your own EP vs UP validation strategy – please contact our UPC ready team – upcready@hgf.com or your usual HGF representative.
This update was prepared by HGF Partner & Patent Attorney Andrew Camenisch.