Healthcare Scanner
Medical technology most patented subject matter at EPO in 2020
June 2021
Pharmaceutical and biotechnology patent applications also make top 10 most patented.
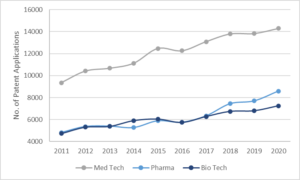
Recent statistics published by the EPO show that a total of 14,295 European patent applications were filed in 2020 in the field of medical technology (MedTech), a rise of 2.6% from the previous year, making it the highest application sector. This is the 9th year in the past decade that MedTech has obtained the top spot, only losing out narrowly to digital communication in 2019. In two other healthcare fields – pharmaceuticals and biotechnology, application numbers were 8,589 and 7,246 putting them in 6th and 8th place respectively. These three technical fields together comprise 16.7% of all patent applications filed at the EPO in 2020.
In this article, we discuss:
- why MedTech patent filings are so high
- which particular areas of MedTech have the highest applications
- the top 10 applicants in the MedTech sector
- the effect of coronavirus on healthcare patent applications.
Why so many healthcare patents?
An ageing population and higher standard of living is no doubt a factor. According to a Deloitte 2020 research paper, the over 65s represent roughly 20% of the western European population and the amount spent on the healthcare sector by developed markets was 77% of the global spend in 2014. The ageing population continues to grow with increased life expectancy and with this so does the market for healthcare products.
Why is MedTech in the lead?
Within the healthcare field, all of medical technology, pharmaceuticals and biotechnology patent application numbers have increased in the past decade. However, the standout performer of 2020 was medical technology with 14,295 applications compared to 8,589 for pharmaceuticals and 7,246 for biotechnology. This may be due to two factors. Firstly, the time to reach the market is generally quicker for MedTech compared to pharmaceutical/biotech products. Research and development time is shorter for MedTech and device improvements are available 18-24 months from previous iterations. Trials for MedTech are typically less time consuming than pharmaceutical trials because medical technology tends to deal with mechanical products which interact with the body in a simpler and more localised way compared to drugs. Secondly the cost to take MedTech products to market is less than pharma and biotech due to more lenient (although still very stringent) regulations.
According to MedTech Europe “more than 500,000 different types of medical devices are produced globally – compared to 20,000 medicinal products”. This goes some way to understanding why more medical technology patent applications are filed compared to pharmaceuticals.
Which Areas of MedTech have the most applications?
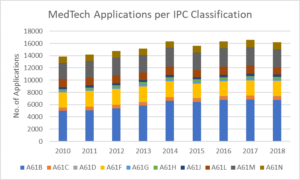
Medical technology can be divided according to the patent classifications listed in the table below. The following bar chart shows that classifications A61B, A61F and A61M were the top three areas for MedTech applications from 2010-2018.
| IPC Classification | Description |
| A61B | Diagnosis; Surgery; Identification |
| A61C | Dentistry; Apparatus Or Methods For Oral Or Dental Hygiene |
| A61D | Veterinary Instruments, Implements, Tools, Or Methods |
| A61F | Filters Implantable Into Blood Vessels; Prostheses; Devices Providing Patency To, Or Preventing Collapsing Of, Tubular Structures Of The Body, E.G. Stents; Orthopaedic, Nursing Or Contraceptive Devices; Fomentation; Treatment Or Protection Of Eyes Or Ears; Bandages, Dressings Or Absorbent Pads; First-Aid Kits |
| A61G | Transport, Personal Conveyances, Or Accommodation Specially Adapted For Patients Or Disabled Persons |
| A61H | Physical Therapy Apparatus, E.G. Devices For Locating Or Stimulating Reflex Points In The Body; Artificial Respiration; Massage; Bathing Devices For Special Therapeutic Or Hygienic Purposes Or Specific Parts Of The Body |
| A61J | Containers Specially Adapted For Medical Or Pharmaceutical Purposes; Devices Or Methods Specially Adapted For Bringing Pharmaceutical Products Into Particular Physical Or Administering Forms; Devices For Administering Food Or Medicines Orally; Baby Comforters; Devices For Receiving Spittle |
| A61L | Methods Or Apparatus For Sterilising Materials Or Objects In General; Disinfection, Sterilisation, Or Deodorisation Of Air; Chemical Aspects Of Bandages, Dressings, Absorbent Pads, Or Surgical Articles; Materials For Bandages, Dressings, Absorbent Pads, Or Surgical Articles |
| A61M | Devices For Introducing Media Into, Or Onto, The Body; Devices For Transducing Body Media Or For Taking Media From The Body; Devices For Producing Or Ending Sleep Or Stupor |
| A61N | Electrotherapy; Magnetotherapy; Radiation Therapy; Ultrasound Therapy |
The effect of coronavirus on healthcare patent applications
The full effect of coronavirus on healthcare patent applications will not be known until at least two years’ time because patent applications are generally first published around 18 months after they have been filed. However, it is highly likely that coronavirus will provide an extra stimulus to increase healthcare patent applications, especially since clinical trials have been expedited given the emergency. In addition the pandemic has provided an opportunity to forge partnerships between universities, SMEs and manufacturers further accelerating patent applications. In other non-healthcare technology, there may well be a downturn in patent applications (as was seen after the 2008 financial crisis) because of increasing financial pressure on businesses, although the “new normal” may offer plenty of opportunities for innovation so the overall effect remains to be seen.
Pharma and biotech in the future
Both pharmaceuticals and biotech have seen significant growth in applications of 10.2% and 6.2% respectively in 2020, more so than medical technology which grew 2.6%. It is likely with the rise of personalised medicines and the advent of new development technology, for example artificial intelligence being used to predict the shape and structure of drug molecules, that the typical trial and error approach of practical lab work can be cut down significantly, which could in turn speed up development and increase the number of patent applications in these two areas.
Top Applicants
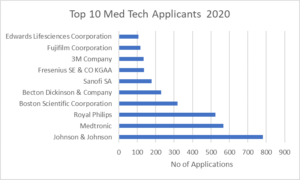
There were some standout performers in the MedTech field. Johnson & Johnson was the top applicant in the MedTech sector with 781 applications overall in 2020. In second place was Medtronic with 567 applications followed by Royal Philips with 524 applications. It is also worth pointing out that Johnson & Johnson also had the 3rd highest number of patent applications in the pharmaceutical field in 2020.
Although patents in the medical technology sector are often used for deterrent or defensive purposes (i.e. to deal with infringers), the primary use of patents is for monetisation of the technology (e.g. via licensing income), particularly for large corporate medical device companies. Patents therefore form an essential part of the MedTech business model which will not be adversely affected by the pandemic and, indeed, will be as important as ever to secure a return on investment in this essential area of technology.
This article was prepared by HGF Professional Standards Officer Dr Edward Pullicino.