Healthcare Scanner
The Internet of Medical Things
June 2021
Exploring the growth of patents for connected medical devices, and how best to protect them.
The Internet of Things (IoT) is expected to change our lives by creating a connected world with billions of devices with embedded sensors and transmission capability exchanging data with other devices and systems over the Internet. Whilst the IoT is well-established in the consumer market, this concept is also frequently employed in medical devices for use in healthcare systems. This trend, known as the Internet of Medical Things, is leading to a paradigm shift in ways of treating some of the harder to reach and pervasive medical problems. Patient access to treatment is improved, regardless of their location.
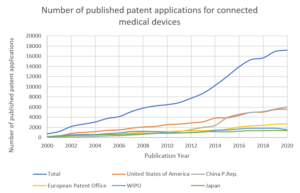
Growth of IP directed to connected medical devices
The digital health industry has expanded exponentially in recent years with the market for medical devices having functionality to enable the exchange of data over the Internet (i.e. “connected medical devices”) predicted to reach USD 52.2 billion in 2022[1]. Common applications of such devices include remote health monitoring and emergency medical notification systems. In order to supplement the increasing desire for domestic healthcare solutions to support aging global populations, demand for wearable medical devices and mobile health monitoring technologies is likely to increase. The use of these devices will be further sustained through enhanced communications technology, including the uptake of 5G mobile networks. Whereas earlier generation mobile networks were limited to the mobile phone industry, 5G is more broadly applicable to other industries, including healthcare. The use of 5G is expected to improve the speed and reliability of data transmission from wearable medical devices, which promises to open up further opportunities to introduce new innovative services.
The emergence of the Internet of Medical Things within the healthcare field has prompted considerable R&D investment in a race to gain market dominance, resulting in a high degree of innovation across various technology sectors. This rapid growth in R&D investment is closely shadowed by the growth in patent applications filed in recent years directed to connected medical devices. The graph below shows approximately a 100-fold increase in patent filings in this field over a 20-year period. This trend appears set to continue. Within this technology field, the top five patent applicants are: Medtronic, Philips, Olympus, Fujifilm and Konica Minolta.
What can be protected?
Consideration of a patent filing strategy should begin with protection for the connected medical device itself, with claims targeting unique features of the device per se. Many connected medical devices are associated with wearable monitoring systems that have data collection, storage, processing and transmission capabilities. Such devices may include biocompatible sensors that can provide real-time continuous measurement of physiological parameters and communicate relevant information to the user, physician or caregiver. Innovative means of forming or incorporating the sensors within a wearable device is one such area that has been subject to a series of patent filings: for instance, the use of flexible substrates and sensor arrays embedded in surgical implants and wound dressings that are adapted to measure and transmit patient specific parameters and biomarkers.
Patent applications may additionally include claims to cover processes of manufacturing the device and methods relating to how the medical device is used, including how data is communicated from the medical device to another part of a system, perhaps for further processing or analysis. The manner in which physiological data from the user is obtained, processed and transmitted by the medical device (or a connected part of the system) may also be protectable. The emergence of Artificial Intelligence (AI) is one particular advancement that has prompted the filing of numerous applications in this domain. Patent protection for inventions implemented through AI is a fast-evolving field. The European Patent Office (EPO) is at the forefront of this trend. The EPO considers that patents may be granted for the use of AI in connected medical devices, for example, the use of a neural network in a heart-monitoring apparatus to identify irregular heartbeats[2].
We also increasingly see patent applications directed to communications security to ensure the protection of any patient data stored, transmitted and/or received by the device. As for any technology concerning the transmission of sensitive data across the internet, privacy is critical. This has become especially important in view of the prominence of General Data Protection Regulation (GDPR) legislation in Europe[3] and the Health Insurance Portability and Accountability (HIPAA) standards adopted in the US[4].
How best to secure patent protection?
In order to secure coverage for different aspects of a connected medical device, a single patent application may include a variety of claim types directed to the apparatus itself, how it is made and used, and the manner in which data generated by the device is processed and used. Although patents may be obtained across a broad range of technology sectors, a number of jurisdictions include exclusions that are intended to restrain the grant of patent rights in certain fields of application.
In Europe, a European patent cannot be granted in respect of methods of treatment of the human or animal body, either by surgery or therapy[5]. The rationale for this patentability exclusion is to provide medical and veterinary practitioners the freedom to use the best available treatments without being inhibited by a worry that such a treatment might be covered by a patent. The law in Europe further stipulates that this exception to patentability does not apply to products for use in such methods. Claims directed to features of a connected medical device would not typically be caught by this exclusion. However, care must be taken where the medical device as claimed is only complete and configured during or after surgical implantation, particularly wherein the device is defined functionally and that function is only configured in vivo. This may be achieved when drafting patent claims by avoiding claiming elements in a manner that would require a surgical or therapeutic step to elicit the function of the device within the body.
Further, in Europe the same provision also excludes diagnostic methods practised on the body. A connected medical device may well generate data suitable for diagnosing a pathological state and may well be positioned on (or in) a patient’s body. Care may need to be taken to avoid falling within this exclusion. For instance, in some circumstances a connected medical device may operate to collect clinical data that could be of diagnostic relevance but could equally be used in some other way, so that the final deductive step of diagnosing a condition is not an essential feature of the invention and method of using the device may be patentable.
As for any invention, a patenting strategy for a connected medical device should consider the range of potential infringers of the patent. A claim directed to the connected medical device, and its manufacture, is likely to be the focus to target rival manufacturers and potentially resellers. While it is unlikely that end users of a connected medical device (patients, physicians and caregivers) are to be targeted, a method of use claim may still have value, for instance where the focus is the transmission of data from the device for processing elsewhere, and that processing could be performed by a commercial organisation. This may be particularly true where the value of the invention lies in the generated clinical data, more than the sensing technology.
Where protection is sought for the transmission of data from a connected medical device, it is desirable to separate the transmission of data from the device, and the reception of that data (and its processing) by a separate apparatus in order to avoid ending up with a claim that can only be directly infringed by multiple parties acting in concert. Further, the two ends of the data transmission may potentially be in different jurisdictions, leading to difficulties in establishing where the infringement takes place that are best avoided.
Typically the inventive part of a connected medical device will be partly or fully implemented in software. In Europe patent protection is excluded for a program for a computer, to the extent that the invention relates to a program for a computer “as such”[7]. However, any computer program (including where embedded in a connected medical device) having a technical effect moving the invention away from a purely abstract concept could be patentable. The identification of irregular heartbeats discussed above is an example of such a technical effect. Returning to inventions utilising AI, both the use of training data to create a trained AI model, and the application of that model to process data from a connected medical device to generate new insights from physiological data may be protected.
Conclusion
Given the size of the market for connected medical devices, the investment required to bring products to market and the relative ease with which many products can be copied, patent protection should be sought. While there are traps for the unwary, valuable protection is available for different aspects of connected medical devices and how they are used.
[1] https://www2.deloitte.com/global/en/pages/life-sciences-and-healthcare/articles/medtech-internet-of-medical-things.html
[2] https://www.epo.org/news-events/in-focus/ict/artificial-intelligence.html
[3] https://ico.org.uk/for-organisations/in-your-sector/health/health-uk-gdpr-faqs/
[4] https://campaigns.hgf.com/healthcare-scanner/issue-1/article-3/
[5] https://www.epo.org/law-practice/legal-texts/html/epc/2016/e/ar53.html
[6] https://www.epo.org/law-practice/legal-texts/html/guidelines/e/g_ii_4_2_1_3.htm
[7] https://www.epo.org/law-practice/legal-texts/html/epc/2016/e/ar52.html
This article was prepared by HGF Patent Directors Richard Gover and Dr Adam Hines.































