Healthcare Scanner
Health-conscious IP strategies
January 2022
Strategies for navigating European patent portfolios in healthcare.
Securing patent protection is critically important for innovative companies operating in the healthcare sector. European patent filings in healthcare, and particularly those in the field of medical technology (MedTech), represent the highest application sector with a total of 14,295 European patent applications filed in 2020[1]. In this article we consider the continued growth of patent activity across the healthcare market and how the European opposition process can be used to weaken the strategic position of companies who dominate the sector with their patent portfolios.
Growth of MedTech IP
As MedTech patent filings grow, so do the number of rights obtained by applicants operating in the healthcare market. In addition to restricting the actions of new entrants in the marketplace, patent portfolios can be monetised to provide licensing income and returns on R&D investment. The graph below (based on statistics from the European Patent Office) shows that the number of MedTech patents granted in Europe has vastly increased over the last ten years, reflecting the expansion of R&D activities throughout this industry.
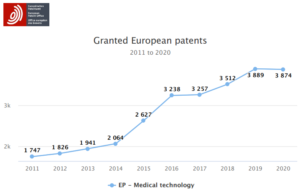
The volume of granted MedTech patents presents a barrier for those seeking entry to the market and also obstructs existing MedTech companies who are looking to expand the scope of their operations. It is not unusual for certain applicants to obtain “patent thickets” that provide an overlapping set of patent rights with the intention of monopolizing a particular technical field. Unless the problematic patents can be nullified, labour intensive “design-around” options may be needed to circumvent each patent. If the claims of the relevant patents are very broad, it may not be possible or commercially feasible to develop an alternative design. In such cases, the only remaining options could be to negotiate a licensing deal with the patent owner(s) or abandon commercialization entirely. Not only therefore, is it important for innovative healthcare companies to obtain patent protection to protect their own R&D, but relevant IP held by competitors must be carefully monitored and kept in check.
Patent watching
An effective risk mitigation strategy requires monitoring the evolving patent landscape and development of competitor IP in and around the relevant technology space. Patent watching services can be employed to track new filings and identify granted patents which could pose a commercial risk. If the claims of the granted patent are considered to be a threat, steps can be taken to challenge the validity of the patent across one or more territories in which the patent is in force.
Patent oppositions in Europe
The most effective way to nullify a patent across Europe is to oppose the patent at the EPO. The outcome of the opposition is effective in all contracting states in which the patent is in force and cannot be appealed nationally.
Recent statistics released by the EPO[2] show that granted patents relating to healthcare have a higher probability of being opposed. The statistics in 2020 indicated a 5.5% and 5.9% opposition rate for biotechnology and pharmaceutical fields respectively, with the average across all fields being 2.4.%. Patents in the MedTech field were just above the average at 2.5%.
An opposition can be filed by any third party within 9 months of the date of grant of a patent. Additionally, the third party can be a “strawman” such that the identity of the true Opponent remains unknown. This can serve as a useful cloaking tactic to avoid alerting the patent owner of the specific relevance of their IP.
Following an opposition, the patent may be maintained as granted, maintained in amended form, or revoked. Statistics from the EPO show that approximately two thirds of cases result in revocation of the opposed patent or maintenance in an amended form. When the patent is amended, the scope of protection covered by the patent is reduced or focused in a particular direction. This may open up further design around possibilities or diminish the relevance of the patent to the existing or envisaged commercial product. An opposing party can therefore obtain a favourable outcome via the European opposition process even if the patent cannot be revoked in its entirety.
How the European patent opposition process works
Timescales
After the filing of an opposition, a party can expect to receive a decision on the outcome of the opposition within 18 months[3] based on the current rate of processing by the EPO.
The decisions issued by the Opposition Division are open to appeal and it is relatively common for parties to appeal the issued decision. At present, the speed of processing by the Opposition Division is not yet matched by the Boards of Appeal with parties often waiting 3 years or more before a final decision is issued following appeal. Recent changes have however been made to the Rules of Procedure at the Boards of Appeal which are intended to expedite the appeal process.
Format
The opposition proceedings are mainly conducted in writing and will culminate in an oral hearing when requested by one or more of the parties. At the hearing, each party presents their case to the EPO with a final decision announced at the end of the oral proceedings. Ordinarily, the oral proceedings will be completed in less than a day but may run for longer in complex cases. In order to minimize disruption during the COVID-19 pandemic which prevented travel to in-person hearings, the EPO launched a pilot project mandating video conference (VICO) as the default for oral proceedings in opposition. To ensure the continued processing of opposition cases, the EPO has extended the pilot project for conducting opposition hearings by VICO until 31 May 2022[4]. Some additional parties may attend an opposition hearing as a member of the public. This can be a useful information gathering tool for parties who are not active participants in the hearing but have commercial interests in the outcome.
Costs
The fee for filing an opposition at the EPO is relatively modest at 815 Euros. The appeal fee for large entities is 2,705 Euros and is reduced to 1,955 Euros for smaller entities such as SMEs and universities. Although the official fees for opposition and appeal are quite low, the majority of costs borne by a party arise from professional or other fees associated with preparing submissions and attending the oral proceedings. However, oppositions at the EPO remain considerably less expensive than patent invalidity proceedings in other jurisdictions such as the US. The recent availability of VICO for European oppositions has also enabled further savings through the reduction of travel costs. Indeed, two thirds of users surveyed by the EPO rated VICO for oral proceedings in opposition positively, one of the major reasons being the reduced travel costs[5].
Summary
The continued expansion of patents rights across the MedTech field in Europe highlights the importance of capitalising on R&D investment through the development of an effective patent strategy. Although innovative companies generally invest considerable resource in establishing their own patent portfolios, it is equally important to restrict or mitigate the commercial impact of granted patent rights held by competitors. The European opposition procedure is a useful instrument available to third parties that can be employed as an accessible, cost-effective mechanism to weaken the strategic position of a dominant patent holder, obtain freedom to operate and avoid litigation, one which many third parties are shown to take advantage of.
This article was prepared by HGF’s Patent Director Dr Adam Hines.
[1] E. Pullicino (June 2021), Medical technology most patented subject matter at EPO in 2020, HGF healthcare scanner
[2] EPO Statistics 2020, President of the European Patent Office
[3] EPO Quality report, section 6.3.2.3 (https://documents.epo.org/projects/babylon/eponet.nsf/0/66A405546212DDF4C12586FC00330A90/$FILE/quality_report_2020_en.pdf)






























